People have been noticing the apparent self-similarity between natural phenomena for thousands of years and grappling with the implications. Even young children will spontaneously use analogy to understand and describe their world using the known to hypothesize on the novel. In science, identifying and exploring these congruencies has lead to fundamental insights but also instances where the apparent and superficial relationships among two phenomena were mistaken for evidence of a more profound connection.
Where is an analogy meaningful and where it is only superficial?
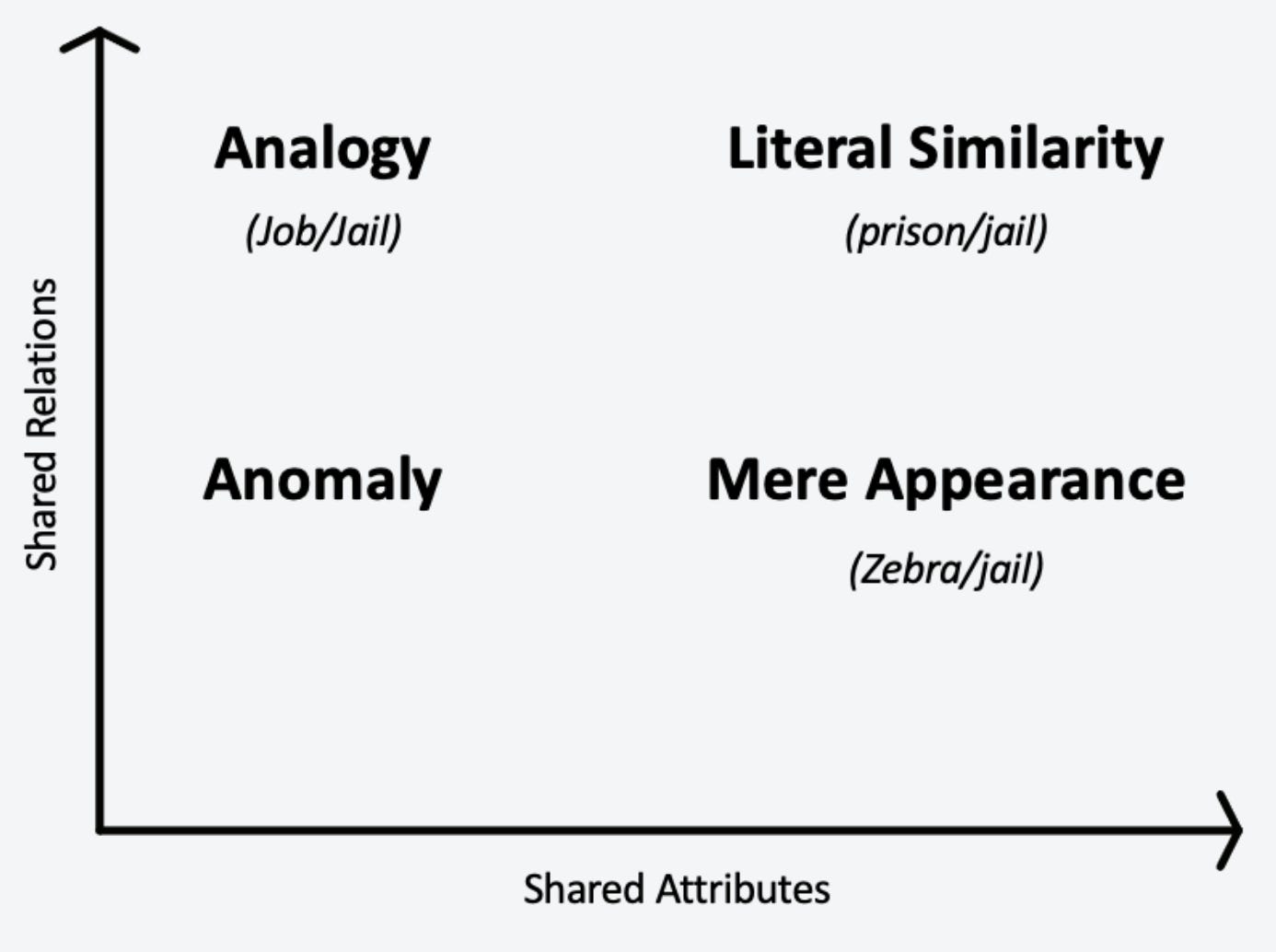
Scholars have conceptualized analogies as occurring in a space of shared relations and shared attributes between the two phenomena in consideration.1 This can be a useful way to consider how analogies can point to hidden structural relationships versus false leads yielding only superficial similarities. Consider the following statement:
“My job is like a jail”
In this statement, if a jail exemplifies a loss of freedom and autonomy, the speaker’s job also has that psychological effect. Jobs and prisons are obviously not similar in other ways and hence they are not literally similar in the way a ‘prison’ and a ‘jail’ are. Yet the relationship between a job and a jail is more interesting than the relationship between old striped prison uniforms and zebras (see in the figure under ‘mere appearance’) - unless there is something meaningful about both old prison uniforms and zebras being striped.2 Interesting analogies exist where shared relations are high, but shared attributes may be limited.
Now let’s apply this framework for understanding analogies to science.
Analogical reasoning in Physics. The case of Kepler and Gravity
In arguing against the prevailing presumption in his day that gravity was a material force, Johannes Kepler (1571-1630) provided the analogue of light:3
“Who I ask, will say that light is something material? Nevertheless, it carries out its operations with respect to place, suffers alteration, is reflected and refracted, and assumes quantities so as to be dense or rare, and to be capable of being taken as a surface where it falls upon something illuminable”
Kepler’s writing shows how scientists have been using analogy and metaphor from the start, bootstrapping the exploration of one scientific idea with another. You can almost imagine Kepler in his frock tweeting this idea out to his followers. Or, if anachronistic imagery doesn’t work for you, at least writing those lines on the wool and animal bonded parchment of his day and giving it to some lad to deliver to a scientific body for a diminutive German coin. But I digress…
Let’s look at another old analogy from physics: the idea that an atom is like the solar system. I’ve diagramed the basic analogy below:
That’s more of less how we understand atoms, right? Take for instance, the logo of the Atomic Energy Commission (1946-1974) or the show the Big Bang Theory. In both instances the central dot is the nucleus surrounded by the orbiting electrons.
Atomic Energy Commission 1946-1974
Formally this idea of an atom being like a solar system or a planet with orbiting satellites was most clearly articulated by Hantaro Nagaoka in 1904. It was dubbed the ‘Saturnian model’ model as it resembled Saturn and its rings. In this model, a large positively-charged atomic nucleus was ringed by negatively charged much lighter electrons.
But..umm…are these analogies correct?
It’s probably unhelpful to consider whether Kepler’s and Nagaoka’s analogies are ‘right’ or ‘wrong’ in an absolute sense. The more relevant questions are:
Were they useful for advancing the science of the day?
Do they still have utility now?
In both cases, the analogies were probably useful for spurring science as they pushed back against the scientific orthodoxy of the day and its limitations. In Kepler’s case, he challenged the notion that gravity had to be composed of a material substance. In doing so, the possibility became open to understanding planetary motion as well as Newtonian gravitation (i.e. ‘action at a distance’) without identifying the ‘stuff’ that intermediated the interaction.
Meanwhile, Nagaoka’s model challenged problems in the prior “Plum pudding model” of the atom as the Plum Pudding model involved (positively charged) protons commingling and embedded with (negatively charged) electrons. Nagaoka recognized these oppositely charged particles would not readily associate and hence needed to be physically separate.4
Whether Kepler and Nagaoka’s conjectures continue to be useful is unclear. We may think of an atom as being like a solar system, but there are numerous points of departure. To name a few:
A solar system is held together primarily by gravity / An atom is primary held together by the strong force
A star is vastly hotter than its surrounding planets / No parallel temperature division exists for an atom
Classical mechanics is generally sufficient to understand the behavior of solar systems / Quantum mechanics is required to understand the behavior of atoms
Kepler’s thinking is similarly problematic. For instance, key to any understanding of light today would include an account of photons including their quantum oddities and speed-of-light constraints.
Consequently, although the analogies were helpful in their day we can become so enthralled with the analogy that we don’t pay attention to the differences. Being enamored with the parallels partially involves not paying attention to their discrepancies!
In subsequent posts, I will look at other powerful analogies in the history of science - including the Gaia Hypothesis and the notion of a ‘tree of life’. Does the history of evolution really resemble a ‘tree’? What has this analogy accomplished? Does it remain useful to science and to the public’s understanding of nature? Where does it mislead?
Thanks for reading.
Gentner, D., and Colhoun, J. (2010). Analogical processes in human thinking and learning. In Towards a theory of thinking (pp. 35-48). Springer Berlin Heidelberg. doi:10.1007/978-3-642-03129-8_3
Early naturalists believed the only purpose of coloration in animals was camouflage. This is a theory that was severely tested when considering zebras, flamingos or many others animals. As Darwin himself realized, some features of organisms are not functional for what we assume is their evident purpose: a peacock’s tail feathers do not aid in flight but peahens find them very becoming!
Gentner, D. (2002). Analogy in scientific discovery: The case of johannes kepler. In N. Nersessian and L. Magnani (Eds.), Model-based reasoning: Science, technology, values (pp. 21-39). New York: Kluwer Academic/Plenum Publisher.
Furthermore, the word “pudding” means such different things to scientists in the North America and the UK and commonwealth countries that it was bound to foster miscommunication! When we talk about the atom being like a pudding, are we thinking a smooth chocolate pudding (North American pudding) or a chunky steak and kidney pudding (UK and Commonwealth countries)? Is the atom fundamentally sweet or savory? It is hard to imagine atomic physics progressing under such a culturally ambiguous metaphor.




I enjoyed this essay, Luke. Super interesting...
I enjoyed this essay, Luke. Super interesting...